Superheavy Elements[]

Relevent concepts: Extended Periodic Table
Due to specific 'magic' numbers of protons and neutrons, some superheavy nuclei exist on an Island of Stability, giving them long enough half lives to have practical uses. There are three distinct islands of stability centered around elements 120 (Weberium), 126 (Andasium) and 164 (Zybanium).
Flerovium[]
Flerovium-298 is the first stable isotope on the first island of stability. Flerovium is very unreactive and so has limited uses. Due to a Flerovium atom's immense size, the metallic bonds formed between atoms are very weak and easy to overcome, leading to a boiling point of -60 degrees. This means Flerovium is an extremely dense gaseous metal at room temperature.
Sorensenium[]


Element 119, Sorensenium (So) is in group 1 of the periodic table and so is an alkali metal with similar properties to the other alkali metals. Sorensenium is named after Søren Peder Lauritz Sørensen; the inventor of the pH scale. This is because Sorensenium hydroxide (SoOH) is the strongest base known to science with a pKb of -1.92. This is strong enough to easily corrode through glass so SoOH must be stored in special beakers with alkaline-resistant coating or simply only produced when needed. Sorensenium is refined from naturally occurring So₂O.
Weberium[]
Drops of Weberium on a watch glass with pipette

Element 120, Weberium (Wb) is in group 2 of the periodic table and so is an alkali earth metal with similar chemical properties to the other alkali earths. However, its chemical properties are not what makes Weberium useful. Weberium is named after Wilhelm Eduard Weber, the electromagnetic physicist. It is so named because Weberium is an excellent conductor of magnetic flux, allowing it either be added to a conventional metal like iron to produce an alloy or just used in its elemental form to make the cores of transformers that can handle much higher electrical power than if other elements are used. These transformers are vital for converting the ultra-high voltage power necessary for operating large gamma-lasers and other high energy applications. Due to the size of the Weberium atom, the metallic bonds it forms are weak. This leads to a very low melting point of -15 degrees, resulting in Weberium taking the form of a pale lavender liquid metal at room temperature.
Andasium[]


An Andasium chain battery of Sanuran design
Element 126, Andasium (An) is the center of the second island of stability. Andasium is very chemically reactive, easily ionizing to An⁺ and further oxidizing up through An²⁺, An⁴⁺, An⁶⁺ and then An⁸⁺. This myriad of different, increasing oxidation states coupled with Andasium's reactivity makes it very useful for making chain batteries when a chemical reaction increases the oxidation number of the Andasium ion progressively, producing a voltage each time. All of these reactions have a standard electrode potential of at least -3.80. Compared to Lithium's single electrode potential of -3.04, this makes Andasium chain batteries better than lithium-ion batteries by at least seven times on the reduction potential alone with advanced electrolytes and architecture able to boost the efficiency even further.
The metallic bonds between Andasium atoms are weak, leading to a very low boiling point in Andasium and so making the metal a gas at room temperature. This, combined with its high reactivity, allows Andasium to be used as a powerful explosive when nuclear or more mundane but less powerful chemical explosives will not suffice. Alternatively, Andasium can to compressed and refined into a liquid crystal form call Ansite. This also contains a lot of chemical energy but will release it more slowly and regularly, allowing Ansite to be used as a fuel, albeit an impractically expensive one. Ansite can be further refined into a metastable solid crystalline form that is very dense. This is used to store Ansite in a small volume so that it can be released later when needed
Bricillium[]

A 1kg Bric Magnet hovering above a piece of pyrolytic carbon.

Element 128, Bricillium (Bc) lies on the second island of stability. Bricilium has unusual atomic properties that result in many unpaired electrons in its outermost electron shell. To make use of this property, Bricillium is added to an alloy of a ferromagnetic metal like iron along with rare earth elements like Gadolinium and other trace elements to produce very powerful permanent magnets that do not easily lose their magnetic properties. These are called Bric Magnets and have a magnetic strength between 2.2 and 2.4 Tesla, much higher than the 0.6-1.4 of a conventional Neodymium magnet. These magnets are strong enough to self-levitate when places over a strongly diamagnetic material such as Bismuth or Pyrolytic Carbon. This property is utilized in that bric magnets are used to make completely frictionless bearings in machines under high mechanical stress. More mundane applications include use in powerful electrical generators and in servo motors and loudspeakers, although the high cost of Bricillium usually means birc magnets are reserved only for when their extreme magnetic strength is absolutely vital to the function of the application.
Meta-Elements[]
Relevent concepts: μMatter
Meta-elements are 'elements' whose atoms contain particles other than the normal Proton, Neutron and Electron. Examples include Muonic atoms that contain Muons in the place of electrons, atoms whose nuclei contain baryons with exotic quark flavours or boud matter/antimatter states.
Alkagen[]

All three isotopes of alkagen
Alkagen is the lightest of all muonic elements, represented by the symbol (Ak). The atom is only composed of one proton and up to two neutrons. Because there is only one proton, there is only one possible electrope of alkagen because only one muon can ever bind to the nucleus. Because there are no electrons present in the atom, it is completely chemically inert. Alkagen has three iostopes, all with different physical and nuclear properties. Because the muon orbits the proton so much closer than in alkagens periodic counterpart hydrogen, alkagen is much denser.
Alkagen-1 is a gas at room temperature but can be easily turned into a gas through moderate levels of pressure. Because of its chemical inactivity, it has few applications in industry except to be converted into other isotopes through neutron bombardment.

An alkagen harvesting ship in a nebula.
Alkagen-2 has a greater density than alkagen-1 due to its greater atomic weight. because of this, it is a liquid at room temperature although it is still seven times less dense than water. because the muon is so close to the nucleus of the μatom, Alkagen-2 is able to fuse at much lower temperatures and pressures than hydrogen.
Alkagen-3 is also a liquid, like alkagen-2. However, it is radioactive and has a half-life of 12 years. Alkagen-3 can fuse with Alkagen-2 at room temperature. This is useful in for such applications as cold-fusion power, propulsion and fusion weaponry and as a result, is a valuable resource for most space-faring species.
Alkagen is by far the most common muonic element. It can be harvested from the nebulae left over from supernovae where in the past, cosmic radiation from the supernova bombarded hydrogen intensely enough to produce stable volumes of it. This is almost entirely alkagen-1 however. In order to obtain the large quantities of alkagen-2 and 3 needed for industrial and commercial application, alkagen-1 is bombarded with neutrons to form the heavier isotopes.
Solareon[]

A volume of solareon. Microfusion events are visible.
Solareon is the second lightest muonic element and is represented by the symbol (So). it contains two protons and between one and two neutrons with solareon-4 being by far the most common isotope. Because of the density that the muon gives it, solareon is a liquid at room temperature. It has a gold color and an almost metallic sheen cause by the way the muon makes the individual atoms absorb and re-emit light. Solareon has two possible electropes, solareon[I] and solareon[II].
Solarium[I] has one electron in its outer shell which makes it behave chemically like hydrogen while being much denser due to its greater number of nucleons. It can be used to make exotic polymers, replacing the less dense hydrogen which forms much denser and stronger materials. These are frequently used in sheild construction and armour.
Solareon[II] has no electrons in the atom, this gives it the same fusional properties as alkagen because of the distance between the nuclei. A sample of solareon will sparkle because of the light that microfusion events between the much less concentrated solareon[II] atoms in the sample emits. Because there are no electrons in the atom, solareon[II] is chemically inert. This makes it not very useful industrially on its own.
Like alkagen, solareon is obtained by collecting it from the nebulae left over after supernovae. However, it is found in much lower concentrations in these nebulae so this is not the primary source for most empires. The main source of solareon for most industrial use is as a byproduct of alkagen cold-fusion. It is produced is large quantities from transmutation reactor built for the purpose of solareon production specifically.
Denarium[]
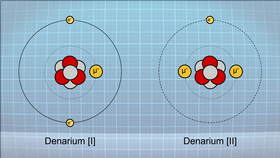
Two electropes of denarium, denarium [I] and denarium [II]
Denarium is a metallic μmatter element represented by the symbol (De) that contains three protons four neutrons in its most common isotope. It is blue in color and has a very high lustre which makes it attractive to many species. The metal is soft and malleable which makes it easy to work into a variety of shapes. Denarium has two stable electropes, deanrium[I] and denarium[II].
Denarium[I] is by far the most common and is what the word "Denarium" colloquially means and because of the arrangement of electrons around the nucleus to account for the presence of the muon, it is completely chemically inert. This property has made it highly valued by those species with the technological capability to acquire it has a method of storing wealth as it is rare enough to be still valuable in such levels of technological advancement (unlike now-common metals like gold) and it will not be lost to corrosion over time. These properties have lead it to be used to manufacture physical currency for many races and bars of denarium are almost universally recognised as a trading commodity.

Bars of denarium
With the addition of an additional muon, denarium[II] has completely different chemical properties to its lighter cousin, like being able to form covalent bonds with non-metals. There is only one electron in its valence shell and so it is very reactive and behaves chemically like hydrogen while having the physical properties of a metal. Its reactivity makes it not as sought after as denarium[I] but there are a few industrial used for this electrope such as in extreme-performance batteries and exotic polymers.
Denarium's rarity comes from the fact that as a muonic element it is not formed by the fusion processes within stars. Instead, denarium is formed by deposits of lithium being bombarded with high-intensity electromagnetic radiation to an intensity that Kalekra stability becomes significant enough to form a stable deposit. Natural sources of radiation intense enough to create these deposits are almost solely limited to gamma-ray bursts and so because of the rarity of these events, finding planets that have been hit and contain denarium is very uncommon.
Glucinium[]

The atomic structure of a glucinium μatom
Glucinium is a metallic μmatter element represented by the symbol (Gc). Glucinium-9 is the most common isotope of glucinium and has 4 protons and 5 neutrons. Glucinium is slightly pink in color with a high lustre. It is a light metal with a relatively low melting point, this makes it very easy to alloy with other metals. It is very reactive in its metallic state and forms salts easily, chemically behaving similarly to lithium.
A key application of glucinium is as glucinium salts. Glucinium chloride (GcCl) is extremely hygroscopic, drawing large amounts of moisture from the air. This makes it extremely useful in the environmental regulation systems of some larger spaceships as humidity controller. More complex molecules such as glucinium stearate (GcO2C(CH2)16CH3) can also have a wide variety of applications such as lubricants that work at very high temperatures. These lubricants are often used in high-speed rotating particle accelerators to reduce the amount of friction generated.
Like denarium, glucinium is produced naturally by gamma-ray burst striking planets with mineral deposits. The difference is that in order to produce glucinium, a beryllium deposit must be irradiated. However, glucinium is more common than denarium because the nucleus of beryllium contains a greater number of protons than that of lithium and so when the muon forms it has a greater chance of binding to the nucleus because of the greater electric attraction.
All muonic elements past Glucinium are either too unstable to too rare to have any practical use and so are not employed in technology and are not traded for.
Minerals[]
Vectrite[]
A deposit of vectrite on Sanura. The atmospheric heat creates sparks.
Native to the planet Sanura, vectrite is a light purple mineral that forms complex, polygonal crystals in extreme heat and pressure environments. It is rich in both platinum and rhodium which gives it thermocouple-like properties, allowing it to convert heat into an electric potential difference. The crystalline structure allows an electric current to be created in the mineral which often creates sparks when the crystal is in a high heat environment. Vectrite will only conduct when in its crystallized form, in this form it is known as vibrant vectrite.
This property allows vectrite to be useful in electrical generators because it means that moving components are not required to convert thermal energy into electric current. As a mineral, it has high melting point and so can be used in direct contact with very hot components, improving the efficiency of generators in which it's used by decreasing energy loss.
Alloys[]
Amaranthium[]

A pile of amaranthium bars.
Amaranthium is a complex metallic-vitreous alloy first produced by the Sanurans used in the construction of spaceship hulls. The metallic part of the alloy is primarily composed of titanium with small amounts of tungsten and rare earths such as neodymium. The crystalline part of the alloys comes from microcrystals of vectrite that give the allow its distinctive purple color. The hulls of Sanuran ships are subjected to tremendous mechanical stress during quantum tunneling and so the hulls must be constructed from an incredibly strong material, of which amaranthium is one. Amarathium owes its strength the the vectrite crystal microstructure. When the metal is subjected to stress, heat is created due to friction. This heat then creates an electric current in the vectrite substructure which in turn creates a strong magnetic field withing the metal that holds it in shape. Because of this, the more stress that amaranthium is subjected to, the stronger it becomes in spite of the strain.
Amaranthium is produced is extremely high-temperature and high-pressure environments to ensure that the vectrite crystallizes into the proper structure. This makes it very expensive so it is almost solely used by the Sanurans because they are the only species with the infrastructure to produce it economically. Because of its stress resistant properties, in order to manipulate amaranthium into useful shapes, it must be cooled down to minimize the effect of thermo-induction and then subjected to a tremendous force to overcome the brittleness that comes with low temperature metals.
Angmallen[]

A pile of angmallen bars.
Glucinium's physical properties make it useful for forming alloys that are light but very strong. The most common glucinium alloy is angmallen, an alloy of titanium, magnesium and glucinium. Angmallen is used mainly alongside other metals in the construction of spaceship hulls. The addition of glucinium makes the alloy much lighter which is important when used in the hulls of surface-to-space shuttlecraft where takeoff weight is an important factor. Its strength also makes it ideal for withstanding the large mechanical and thermal strain put on spaceship hulls during superluminal travel.
Notes[]
Trivia[]
- Elements 130-136 (Lucrium, Gulium, Superbium, Desidium, Luxurium, Invidium and Irium) are named after the seven deadly sins translated into Latin.
- Elements 145-149 (Maatium, Edenium, Olympium, Purusharium and Yggdrasilium) are named after cosmological myths in all the major world religions or major past religions.
- Elements 154-162 (Newtonium, Galileum, Darwinium, Hawkinium, Pasteurium, Teslium, Faradium, Mendelium and Alcubium) are all named after famous scientists that are not currently recognised on the actual periodic table.
- Zybanium is named after a fiction metal from the extended Dr Who universe of the same name.


